The Future of Aesthetic Treatments: A New Contender Emerges
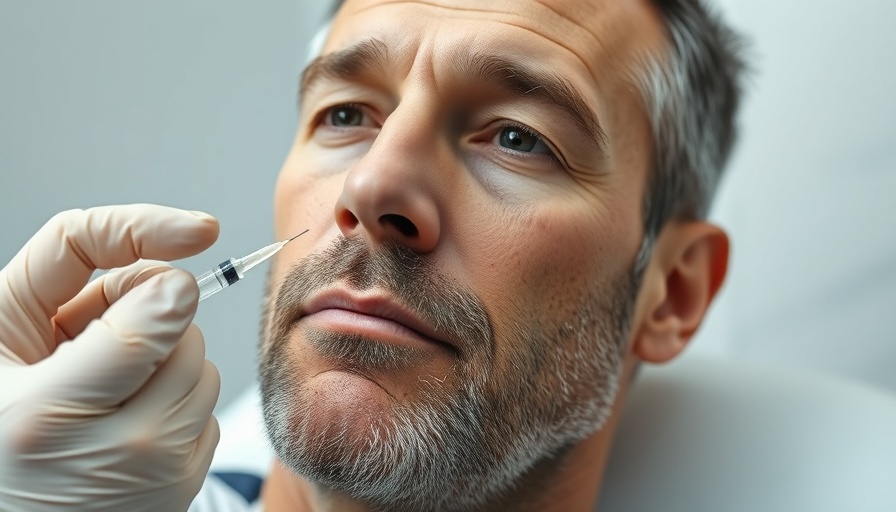
As more individuals seek cosmetic procedures to enhance their appearance, the demand for innovative, effective treatments continues to grow. Allergan Aesthetics, a subsidiary of AbbVie, recently took a significant step in this evolving landscape by submitting a Biologics License Application (BLA) for trenibotulinumtoxinE (TrenibotE), aimed specifically at treating moderate to severe glabellar lines, commonly known as frown lines.
Transforming Aesthetic Experiences
TrenibotE, a serotype E botulinum toxin, is set apart by its rapid onset and shorter effects compared to traditional neurotoxins. Patients may experience results as early as eight hours after administration, with an efficacy period typically lasting two to three weeks. Dr. Darin Messina, senior vice president for aesthetics R&D at AbbVie, suggests that this novel treatment could redefine the experience for newcomers to aesthetic procedures. This is particularly crucial as many patients express concern about the potential for unnatural appearances, a prominent barrier to entry in cosmetic enhancements.
Understanding Glabellar Lines: A Common Concern
Glabellar lines present a common aesthetic concern among various demographics, often tied to aging and repeated facial expressions. The psychological impacts of these lines can weigh heavily on an individual's self-esteem and confidence, prompting many to consider intervention. With new treatments coming to market, patients have a wealth of options to explore, giving rise to a more informed generation of consumers who prioritize natural-looking outcomes.
Clinical Trial Potentials: Insights from Research
The BLA submission is backed by extensive clinical data from over 2,100 patients involved in pivotal Phase 3 trials (M21-500 and M21-508) and an open-label safety study (M21-509). All primary and secondary endpoints were successfully met, reporting safety profiles that remained comparable to placebo even after multiple treatments. This reinforces the potential of TrenibotE to not only meet aesthetic needs but also to maintain clinician and patient trust through its safety assurances.
Addressing Patient Concerns: Building Confidence
Dr. Cheryl Burgess, lead investigator for one of the pivotal Phase 3 studies, highlighted that one of the prevailing barriers to treatment is the fear of an unnatural outcome. With the introduction of TrenibotE, patients are offered a chance to ease into the experience of aesthetic treatments. As shorter duration results provide a lower-risk entry point, patients can experiment with their appearance without the fear of making a long-term commitment, essentially empowering them to explore their aesthetic preferences more freely.
Gearing Up for the Launch: What to Expect
As the aesthetic landscape continues to shift, industry professionals are keenly watching the development of trenibotulinumtoxinE and its implications for practices and patient experiences. With growing competition in the aesthetic domain, the introduction of innovative products like TrenibotE may challenge existing solutions, pushing manufacturers to further refine their offerings. This ongoing evolution presents a promising horizon for patients seeking effective yet non-permanent solutions.
Embracing Aesthetic Innovation: Opportunities Ahead
As we move forward, those in the aesthetic community must remain attuned to the changing needs and preferences of patients. The anticipated launch of trenibotulinumtoxinE not only reflects the scientific advancement in treatment modalities but also aligns with a broader cultural shift toward empowerment and personal choice in aesthetic enhancement. Patients can look forward to a future where they have more control over their aesthetic journeys.
In conclusion, with the BLA submission of trenibotulinumtoxinE, Allergan Aesthetics is not just presenting a new product but appears to be carving a pathway for a more confident approach to aesthetic treatments.
 Add Row
Add Row  Add
Add 






Write A Comment